Definition:Acid Dissociation Constant (Ka) is a quantitative measure of the strength of an acid in solution. It is the equilibrium constant for the dissociation reaction of an acid into its conjugate base and a hydrogen ion (H+).
Click the Translate button(see right) on this post to set your Own Language to understand more perfectly!!
Acid Dissociation Constant Calculator
Definition Continue: Acid Dissociation Constant (Ka)
Acid Dissociation Constant (Ka) is a quantitative measure of the strength of an acid in solution. It is the equilibrium constant for the dissociation reaction of an acid into its conjugate base and a hydrogen ion (H+).
Key Factors:
Acid Concentration (mol/L): The molarity of the acid solution, representing the number of moles of acid per liter of solution. (e.g., 0.1 M acetic acid)
Concentration of Ions (mol/L): The molarity of the dissociated ions, specifically the hydrogen ion (H+) and the conjugate base concentration. (e.g., [H+] = 1.34 x 10^-3 M)
The Dissociation Equation:
For a generic weak acid, HA:
HA <=> H+ + A-
The acid dissociation constant, Ka, is expressed as:
Ka = [H+][A-] / [HA]
where:
[H+] is the concentration of hydrogen ions
[A-] is the concentration of the conjugate base
[HA] is the concentration of the undissociated acid
Example Calculation:
Let's calculate the Ka for acetic acid (CH3COOH) given the following information:
Initial concentration of acetic acid = 0.1 M
Equilibrium concentration of H+ = 1.34 x 10^-3 M
Since acetic acid is a monoprotic acid, [H+] = [A-]
Ka = (1.34 x 10^-3 M) * (1.34 x 10^-3 M) / (0.1 M - 1.34 x 10^-3 M)≈ 1.8 x 10^-5
Note:
The equation you provided (kw = acidConcentration * ionConcentration) is incorrect. Kw is the ion product of water and is used in different contexts, primarily for calculating pH and pOH.
The calculation of Ka often involves simplifying assumptions, such as assuming that the change in acid concentration is negligible compared to the initial concentration.
For weak acids, Ka values are typically small, indicating a low degree of dissociation.
Stronger acids have larger Ka values, indicating a higher degree of dissociation.
Additional Considerations:
Temperature: Ka values are temperature-dependent.
Ionic Strength: High ionic strength can affect Ka values due to the influence of other ions on the solution.
Activity Coefficients: For more accurate calculations, activity coefficients can be introduced to account for deviations from ideal behavior.
By understanding the concept of Ka and its calculation, you can assess the strength of acids and make predictions about their behavior in solution.
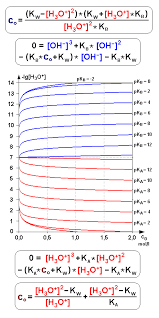
The Acid Dissociation Constant (Ka) is a quantitative measure of an acid's strength in a solution, representing the equilibrium constant for the acid's dissociation with water to form hydronium ions (H3O+) and its conjugate base. The formula for Ka is:
𝐾𝑎=[𝐻3𝑂+][𝐴−][𝐻𝐴]
Ka= [HA][H3O + ][A − ]
Where:[𝐻3𝑂+] is the concentration of hydronium ions,
[𝐴−] is the concentration of the conjugate base,
[HA] is the concentration of the undissociated acid.
Solvent Effects on Ka
The choice of solvent significantly affects the Ka value. Generally:
Polar solvents (like water) enhance acid dissociation, resulting in higher Ka values.
Non-polar solvents decrease acid dissociation, leading to lower Ka values.
Examples with Acetic Acid (CH3COOH)
Water: Acetic acid dissociates well, with a Ka of approximately 1.8×10−5
.
Ethanol: As a less polar solvent, ethanol results in a lower Ka for acetic acid compared to water.
Acetone: Being even less polar than ethanol, acetone further reduces acetic acid dissociation, resulting in a significantly lower Ka.
Factors Affecting Ka in Different Solvents
Dielectric Constant: Higher values favor ion solvation, increasing Ka.
Solvent Polarity: Polar solvents generally yield higher Ka than non-polar ones.
Solvent Basicity: Basic solvents may react with the acid, affecting its dissociation.
Temperature: Changes can influence the equilibrium constant and thus the Ka value.
Practical Implications
Understanding how solvents affect Ka is important in fields such as:
Chemistry: For selecting solvents in acid-base reactions, titrations, or extractions.
Pharmacology: To determine drug solubility and bioavailability in different environments.
Environmental Science: To evaluate how acids behave under various environmental conditions.
How is it possible to earn money using the knowledge of Acid Dissociation Constant Calculation in real-life applications??????
Understanding acid dissociation constants (Ka) can lead to various career opportunities in several industries. Here’s a concise overview of how this knowledge can be applied:
1. **Chemical Industry**:
- **Process Optimization**: Use Ka to improve reaction yields and efficiency.
- **Product Development**: Involves selecting reactants and controlling conditions for acid-base reactions.
- **Quality Control**: Maintain optimal pH levels for product quality.
2. **Pharmaceutical Industry**:
- **Drug Development**: Formulate drugs and predict solubility based on their acid-base properties.
- **Drug Stability**: Develop methods to stabilize drugs using Ka values.
3. **Environmental Science**:
- **Water Treatment**: Determine water acidity and select treatment methods.
- **Soil Remediation**: Use Ka in developing strategies to fix soil pollution.
4. **Food and Beverage Industry**:
- **Food Processing**: Control pH to enhance flavor and prevent spoilage.
- **Beverage Production**: Optimize stability and taste through pH control.
5. **Academia and Research**:
- **Teaching and Research**: Opportunities in universities that involve Ka calculations.
- **Consulting**: Provide expertise in acid-base chemistry to different industries.
6. **Analytical Chemistry**:
- **Quality Control**: Utilize Ka for techniques like titrations and pH testing.
- **Method Development**: Create new analytical methods based on acid-base equilibria.
In summary, mastering acid dissociation constants offers a strong foundation for various chemistry-related careers, enabling contributions to innovation and quality across multiple sectors.
Do YOU Want To Earn Money In Various Ways, Click The Link & Explore Your Field of Interest!!!








No comments:
Post a Comment